News
Adaptable polyurethane networks containing tertiary amines as intrinsic bond exchange catalyst
12.06.2024
Lars Schwarzer, Seema Agarwal
Macromol. Chem. Phys. 2024, https://doi.org/10.1002/macp.202400072
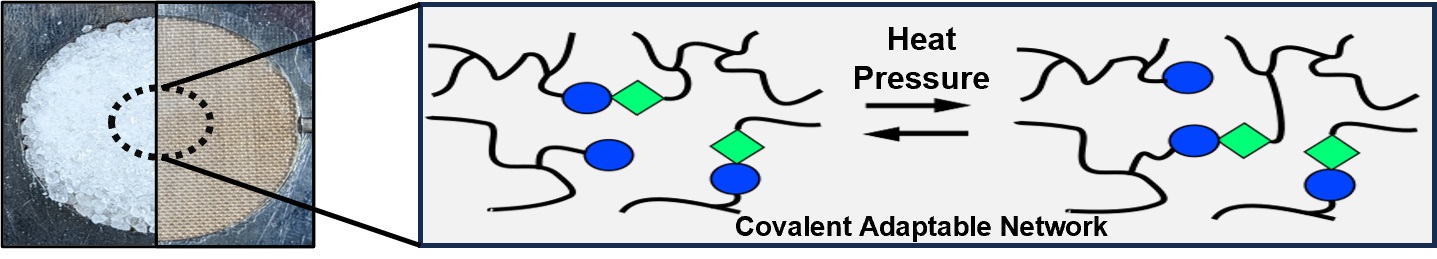
Vitrimers exhibit unique properties, such as thermal recyclability akin to thermoplastics, while structurally mirroring thermosets in terms of strength, durability, and chemical resistance. However, a significant limitation of these materials is their dependence on an external catalyst. Consequently, this research aims to develop vitrimer materials that incorporate an intrinsic catalyst, thus maintaining excellent thermomechanical properties and recyclability. Polyaddition polymerization is employed to synthesize the desired polymer, incorporating a self-synthesized tertiary amine unit, (bis(2-hydroxyethyl)-3,3′-((2-(dimethylamino)ethyl)azanediyl)dipropanoate) (N-diol), as an internal catalyst for transcarbamoylation and potential transesterification reactions. The resulting polymer, with a gel content of 97% and a glass transition temperature of 29 °C, is fabricated into test samples for comprehensive thermal and mechanical evaluations. The material demonstrates an initial Young’s modulus of 555 MPa, retaining 81% of this value after two recycling processes. Additionally, using stress relaxation analysis (SRA), a topology freezing temperature of 82 °C, indicative of the characteristic Arrhenius-like relaxation behavior, is identified with a bond exchange activation energy of 163 kJ mol−1.